Abstract
Background
For AML patients (pts) unfit for induction, outcome with hypomethylating agent (HMA) therapy is enhanced by the addition of venetoclax (VEN), making this dual treatment their current standard of care. A randomized phase II study (DECIDER) showed that the combination of decitabine (DAC) with all-trans retinoic acid (ATRA) also leads to a significantly improved objective response rate and overall survival (OS) in newly diagnosed elderly AML pts - without added toxicity - compared to DAC without ATRA1. Activity of the combination was also observed with adverse genetics, including TP53 mutations (TP53mut)2. Mechanistically, we demonstrated cooperative effects of both drugs on global gene regulation and chromatin remodelling in AML cell lines3. Notably, ATRA has an important role in normal hematopoietic percursors by preserving stemness4, and in one study enhanced the antileukemic activity of VEN in vitro5. We now asked whether cooperation between DAC and ATRA can also be observed in PDX mouse models of AML, and whether antiproliferative and proapoptotic effects are further enhanced in the triple combination of DAC+ATRA+VEN.
Materials and Methods: The 5 AML cell lines MV4-11, MOLM-13, OCI-AML3 (all TP53wt), U937 and KG-1 (both TP53mut) were treated with 100 nM DAC, 1 µM ATRA or 100 nM VEN (single agents, dual and triple combinations) over 72 hours. Proliferation and viability were assessed every 24h (Luna cell counter), apoptosis at 96h and 120h by Caspase 3/7 Glo assay. Western blots were performed at 120h (U937, OCI-AML3) to quantify Bcl-2, Mcl-1 and Bcl-xl. Two PDX models were generated (one pt with de novo AML and normal karyotype, one with sAML from MDS and complex-monosomal karyotype) as previously described6. Mice were treated with DAC for 5 days (1 mg/kg i.p.), ATRA (s.c. 0.5 mg for 10 days) or the combination of both (5 animals per cohort). Treatment was started once a tumor size of >100 mm³ was reached to ensure successful engraftment.
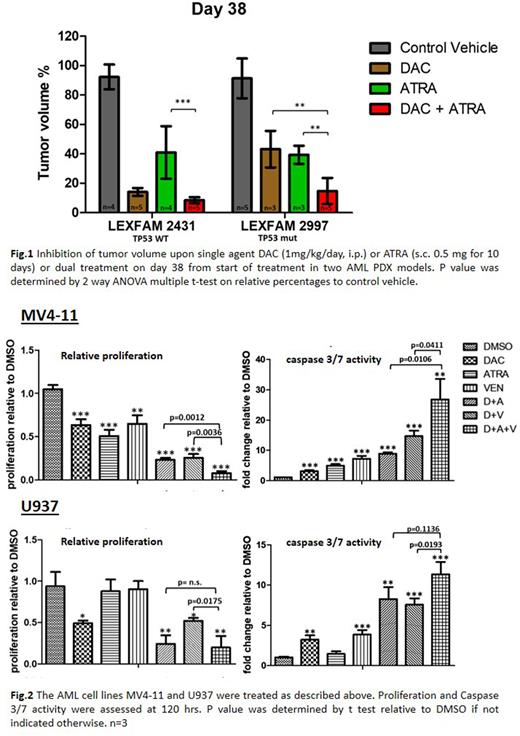
Results: In two AML PDX models, DAC treatment inhibited tumor growth by 86% / 57%, ATRA treatment by 59% / 61%, and combined treatment of DAC+ATRA by 92% / 85% (the combination being significantly more effective than ATRA alone, Fig. 1). Antileukemic activity of both single-agent treatments as well as the combination was observed irrespective of TP53 mutational status. Utilizing a treatment scheme for AML cell lines, established concentrations of DAC, ATRA and VEN were used to determine effects of single, dual and triple treatment upon growth, viability and apoptosis induction.
Here, the ATRA+VEN combination showed cooperative effects by inducing apoptosis, and reducing the proliferation rate as well as viability in 3 TP53wt cell lines (but not in the 2 TP53mut lines). Compared to dual treatments, the addition of ATRA to DAC+VEN resulted in stronger inhibition of proliferation and reduced viability, through an increased apoptosis rate as measured by Caspase 3/7 Glo assay. (Fig. 2: MV4-11, U937). No antagonization was observed. By western blot, the addition of ATRA (alone and in combination) led to downregulation of Bcl-2 (U937 and OCI-AML3 cells), supporting a mechanism for the observed enhanced VEN effect when combined with ATRA.
Conclusions
By use of PDX models treated with DAC and ATRA, we could confirm and extend results generated with AML cell lines, and clinically with the response and OS improvement of elderly AML pts observed in the DECIDER trial. Triple treatment with DAC+ATRA+VEN resulted in overall cooperative effects, with additive effects also observed in TP53mut AML cell lines. A placebo-controlled phase III trial in non-fit AML pts studying the addition of ATRA to DAC+VEN is being initiated (DECIDER-2, EudraCT No. 2020-005495-36). Further studies of the pro-apoptotic effects of ATRA e.g. by dynamic BH3 profiling are warranted.
1. Lübbert M, Grishina O, Schmoor C, et al. J Clin Oncol. 2020;38:257-270.
2. Becker H, Schmoor C, Grishina O, et al. Blood. 2021;138(Suppl. 1):2380-2380.
3. Meier, Greve, Zimmer, Bresser et al. Blood Cancer J. 2022, in press
4. Schönberger K, Obier N, Romero-Mulero MC et al. Cell Stem Cell. 2022;29(1):131-148.e10
5. Li D, Liu S, Chen L et al. Blood. 2019;134(Suppl. 1):5055-5055
6. Schueler J, Greve G, Lenhard D et al. Cancers. 2020;12(5):1349
Disclosures
Becker:Novartis: Honoraria; BMS: Honoraria; Servier: Honoraria; Pierre Fabre: Honoraria; Roche: Honoraria. Lübbert:Otsuka: Consultancy; Cheplapharm: Other: Study drug; Syros: Consultancy; Janssen: Research Funding; Astex: Honoraria; Abbvie: Honoraria.
OffLabel Disclosure:
all-trans retinoic acid (ATRA) is commonly used in combination with arsenic trioxide (ATO) in APL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal